A. Romano¹ ,² , N. L. Parrinello ², ³ , ⁸, V. Simeon ⁴ ,⁵ , ⁸, F. Puglisi ², P. La Cava ², C. Bellofiore ¹, ²,
C. Giallongo ², G. Camiolo ², F. D’Auria ⁴, V. Grieco ⁴, F. Larocca ⁴, A. Barbato ², D. Cambria ²,
E. La Spina ⁶, D. Tibullo ⁶, G. A. Palumbo ², ³, C. Conticello ², P. Musto ⁴, ⁷ & F. Di Raimondo ¹, ²*
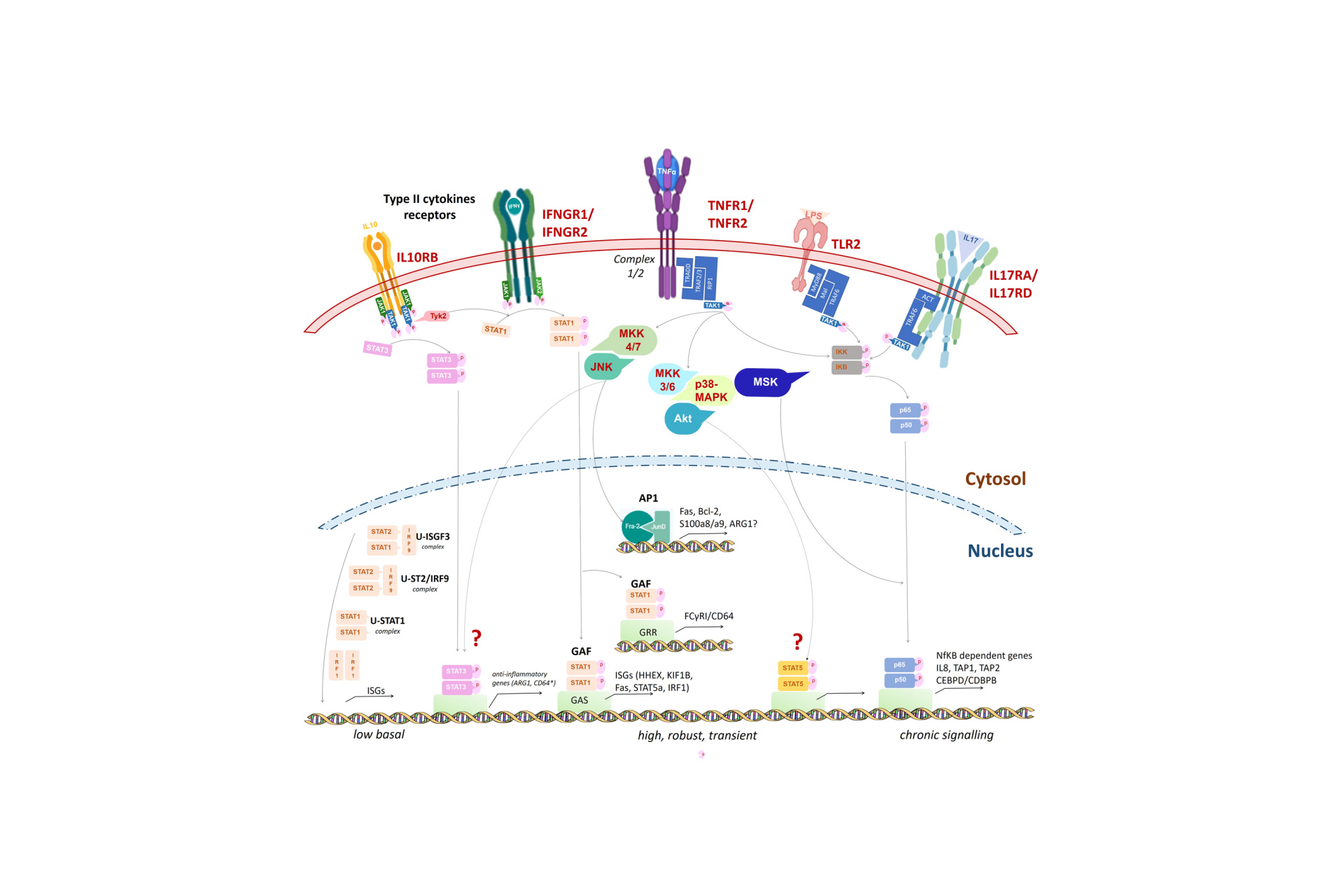
To understand neutrophil impairment in the progression from MGUS through active MM, we investigated the function of mature, high-density neutrophils (HDNs), isolated from peripheral blood. In 7 MM, 3 MGUS and 3 healthy subjects by gene expression profile, we identified a total of 551 upregulated and 343 downregulated genes in MM-HDN, involved in chemokine signaling pathway and FC-gamma receptor mediated phagocytosis conveying in the activation of STAT proteins. In a series of 60 newly diagnosed MM and 30 MGUS patients, by flow-cytometry we found that HDN from MM, and to a lesser extend MGUS, had an up-regulation of the inducible FcγRI (also known as CD64) and a down-regulation of the constitutive FcγRIIIa (also known as CD16) together with a reduced phagocytic activity and oxidative burst, associated to increased immune-suppression that could be reverted by arginase inhibitors in co-culture with lymphocytes. In 43 consecutive newly-diagnosed MM patients, who received first-line treatment based on bortezomib, thalidomide and examethasone, high CD64 could identify at diagnosis patients with inferior median overall survival (39.5 versus 86.7 months, p = 0.04). Thus, HDNs are significantly different among healthy, MGUS and MM subjects. In both MGUS and MM neutrophils may play a role in supporting both the increased susceptibility to infection and the immunological dysfunction that leads to tumor progression.